- 微生物及免疫學研究所 副教授
- 專長領域:病毒學 、EB病毒、流行性感冒病毒
- 辦公室位置: 生物醫學大樓(R408)
- TEL (辦公室): +886-2-28267180
- TEL (實驗室): +886-2-28267000 ext 65625
- E-mail: cjchen@nycu.edu.tw
- ORCID: 0000-0001-8348-2911

學歷
國立台灣大學 植物病蟲害學系學士
美國密西根州立大學 遺傳學學程博士
The Wistar Institute (美國賓州大學) 博士後研究員
經歷
國立陽明大學 微生物及免疫學研究所 副教授
國立陽明大學 微生物及免疫學研究所 助理教授
獲獎榮譽
國家衛生研究院,博士後研究學者 (2001-20020)
陽明大學優良教師之教學優良獎 (97,103 學年度)
醫學系學生網路評鑑優良教師 (92,93,96,97, 98, 99, 100, 101, 102, 103, 110 學年度)
良師益友獎 (108學年度)
論文
- Chen, Chi-Ju, D. J. Leisy, and S. M. Thiem. 1996. Physical map of Anagrapha falcifera nuclear polyhedrosis virus genome. Journal of General Virology 77: 167-171.
- Chen, Chi-Juand S. M. Thiem. 1997. Differential infectivity of two Autographa californica nucleopolyhedrovirus mutants on three permissive cell lines is the result of lef-7 deletion. Virology 227: 88-95. LINK
- Chen, Chi-Ju. 1997. Ph. D dissertation: Characterizations of baculovirus genes that influence infectivity in insect cell lines and larvae. Michigan State University
- Chen, Chi-Ju, M. E. Quentin, L. A. Brennan, C. Kukel, and S. M. Thiem. 1998. Lymantria dispar Nucleopolyhedrovirus hrf-1 expands the larval host range of Autographa californica nucleopolyhedrovirus. Journal of Virology 72: 2526-2531.LINK
- Zerby, D, C-J Chen, E. Poon, D. Lee, R. Shiekhattar, and P. M. Lieberman. 1999. The amino-terminal C/H1 domain of CREB binding protein mediates Zta transcriptional activation of latent Epstein-Barr virus. Molecular and Cellular Biology 19: 1617-1626. LINK
- Chen, Chi-Ju, Z. Deng, A. Kim, G. Blobel, and P. M. Lieberman. 2001. Stimulation of nucleosome-directed histone acetylase activity of CBP by transcriptional activators. Molecular and Cellular Biology 21:476-487. LINK
- Deng, Z.*, Chi-Ju Chen*, D. Zerby, H. Delecluse, P. M. Lieberman. 2001. Identification of acidic and aromatic residues in the Zta activation domain essential for Epstein-Barr virus reactivation. Journal of Virology 75: 10334-10347. (* co-first authors). LINK
- Z., L. Lezina,Chi-Ju Chen, S. Shtivelband, W. So, and P. M. Lieberman. 2002. Telomeric proteins regulate episomal maintenance of Epstein-Barr virus origin of plasmid replication. Molecular Cell 9: 493-503. LINK
- Z., C-J Chen, M. Chamberlin, F. Lu, G. A. Blobel, D. Speicher, L. A. Cirillo, K. S. Zaret, and P. M. Lieberman. 2003. The CBP bromodomain and nucleosome targeting are required for Zta-directed nucleosome acetylation and transcription activation. Molecular and Cellular Biology 23:2633-2644. LINK
- Hsu, CH., MD Chang, KY Tai, YT Yang, PS Wang,C-J Chen, YH Wang, SC Lee, CW Wu, and LJ Juan. 2004. HCMV IE2-mediated inhibition of HAT activity downregulates p53 function. EMBO J. 23:2269-2280. LINK
- Patel JH, Y. Du, PG Ard, C, Phillips, B Carella, C-J Chen, C. Rakowski, C. Chatterjee, PM Lieberman, WS Lan, GA Blobel, SB McMahon. 2004. The c-MYC Oncoprotein Is a Substrate of the Acetyltransferases hGCN5/PCAF and TIP60. Molecular and Cellular Biology 24:10826–10834 LINK
- Liu, SJ, CH Leng, SP Lien, HY Chi, CY Huang, CL Lin, WC Lian, Chi-Ju Chen, SL Hsieh, P Chong. 2006. Immunological Characterizations of the Nucleocapsid Protein Based SARS.Vaccine Candidates. Vaccine 24: 3100-3108. LINK
- Ho, CH, CF Hsu, PF Fong, SK Tai, SL Hsieh, and Chi-Ju Chen*. 2007. Epstein-Barr Virus Transcription Activator Rta Upregulates Decoy Receptor 3 Expression by Binding to its Promoter. Journal of Virology 81: 4837–4847 (*corresponding author)LINK
- Ho, CH, CL Chen, WY Lee, and Chi-Ju Chen*. 2009. Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis 30:1443-1451 (*corresponding author) LINK
- Tsai SC, SJ Lin, PW Chen, WY Luo, TH Yeh, HW Wang, CJ Chen, and CH Tsai. 2009. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 114: 109-118 LINK
- Tempera, Italo, Zhong Deng, Constandache Atanasiu, Chi-Ju Chen, Maria D’Erme, and Paul M. Lieberman. 2010. Regulation of Epstein-Barr virus OriP replication by Poly(ADP-Ribose) polymerase 1. Journal of Viroloty 84: 4988-4997.LINK
- Cheng, Yi-Ying, Shih-Rong Yang, Ying-Ting Wang, Yu-Hsing Lin, andChi-Ju Chen*. 2017. Amino acid residues 68-71 contribute to influenza A virus PB1- F2 protein stability and functions. Frontiers in Microbiology 8: article 692 doi.org/10.3389/fmicb.2017.00692 (*corresponding author) LINK
- Mei-Yin Liu, Wei-Kai Hua, Yi-Ying Chiou, Chi-Ju Chen, Chao-Ling Yao, Yi-Ting Lai, Chao-Hsiung Lin, and Wey-Jinq Lin. 2019. Calcium-dependent methylation by PRMT1 promotes erythroid differentiation through the p38a MAPK pathway. FEBS Letters Sept.
- Yu-Siang Su, Pei-Yu Hsieh, Jun-Syuan Li, Ying-Hsuan Pao, Chi-Ju Chen, and Lih-Hwa Hwang. 2020. The Heat Shock Protein 70 Family of Chaperones Regulates All Phases of the Enterovirus A71 Life Cycle.Frontiers in Microbiology 11: article 1656. LINK
- Yu-Siang Su, Lih-Hwa Hwang*, Chi-Ju Chen*.Heat shock protein A6, a novel HSP70, is induced during enterovirus A71 infection to facilitate internal ribosomal entry site-mediated translation. Frontiers in Microbiology12: article 664955. (*corresponding author). LINK
實驗室介紹
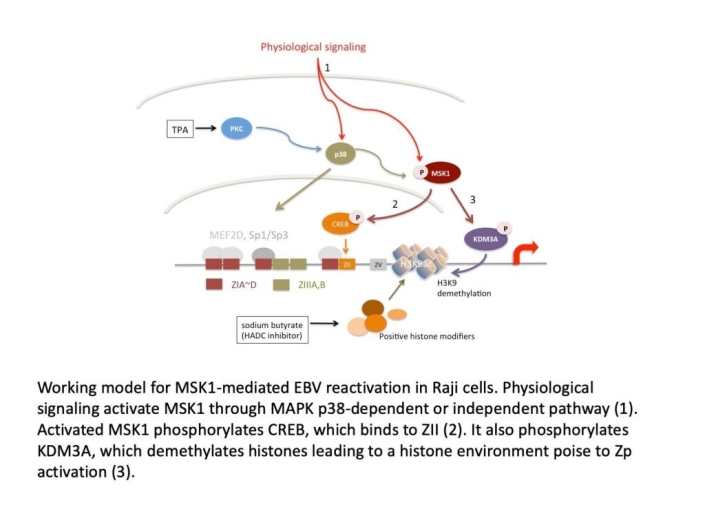
Epstein-Barr virus (EBV) infection causes cancers including Burkitt lymphoma, Hodgkin’s disease,nasopharyngeal carcinoma, and certain types of gastric cancers. EBV establishes a latent infectionright after infection in the form of chromosomal episome, whose genes are mostly repressed, in Bcells and possibly epithelial cells in nasopharynx and oropharynx. The latency can be induced into aproductive infection namely lytic cycle. These two life cycles are interchangeable. People havebeen studying EBV for its association with cancers for a long time. Ample amounts of data suggesthow EBV plays an important role in tumorigenesis. At the same time, new findings regarding itsrole in tumorigenesis are still emerging. However, there are still many unknown. EBV infects tumorcells latently, but high levels of EBV specific antigens and viral titer are high risk factors for EBV-associated malignancies, suggesting genome maintenance and reactivation frequency are bothimportant. We have recently found that mitogen- and stress-associated protein kinase 1 (MSK1)plays an important role in EBV reactivation in Raji cells and EBV maintenance in Akata cells. Weshowed that MSK1 is phosphorylated upon reactivation induced by TPA and sodium butyrate.Activated MSK1 in turn phosphorylates CREB to activate transcription of immediate early gene BZLF1 (Zta) and induces EBV into lytic cycle. We, on the other hand, do not know much abouthow MSK1 plays in maintaining EBV genome in Akata cells.
This proposal focuses on mechanistic studies of MSK1 functions in EBV reactivation and genomemaintenance. Three specific aims are:
- Investigate if MSK1 regulates EBV reactivation is solely through CREB phosphorylation.
- Investigate how MSK1 affects maintenance in three aspects including (1) genome replication, (2) genome partition, and (3) genome establishment.
- Investigate cellular signaling pathways involved in MSK1 activation as well as its role in physiological stimuli known to activate EBV.
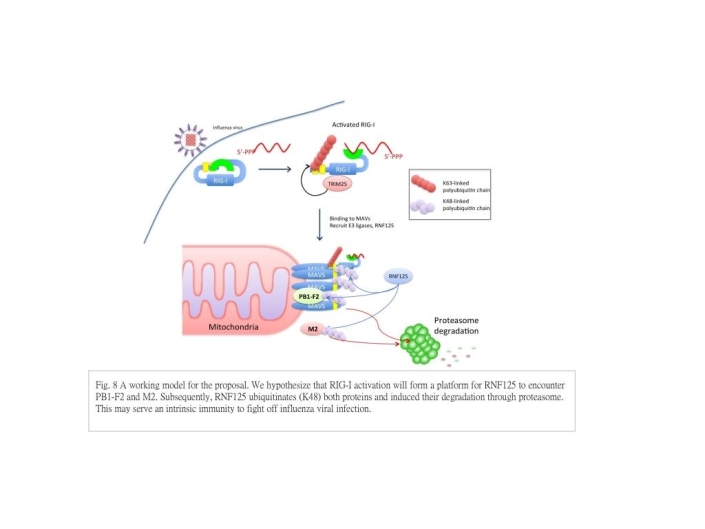
2. RIG-I-induced infuenza protein degradation
- This study aims to understand the stability and degradation of two influenza viral proteins, pro-apoptotic PB1-F2 and ion channel forming M2. Half-lives of proteins are an important aspect of protein functions, however, not much effort was put into this area inthe field of virology.We hope to demonstrate half-lives of proteins and innate immunity-induced proteolysis influence viral protein functions and may affect host-specificity of viral proteins.
Influenza virus (IAV) is a highly contagious respiratory pathogen. Type I interferon is a crucial innate defense against IAV infection. RIG-I (retinoic acid-inducible gene 1) signaling pathway plays an important role to initiate interferon responses in IVA-infected cells. We found that RIG-I activation induced protein degradation of PB1-F2 and M2, and RNF125, a ubiquitin-E3 ligase and negative regulator for RIG-I, may be responsible. We believe it is part of host defense mechanism against influenza virus infection. We hypothesize that RIG-I activation give rise to a platform for RNF125 to encounter PB1-F2 and M2 to add poly-ubiquitin chains to them. As a consequence ubiquitinated viral proteins are sent to proteasome for degradation. Using pharmacological inhibitors to screen the possible pathways involved in RIG-I induced viral-protein degradation, we found that both autophagic and lysosomal inhibitors increased the presence of viral protein, suggesting the involvement of autophagy-mediated proteolysis. Interestingly our preliminary data shows that mitophagy, an autophagic process used to remove damaged mitochondria, can mediate protein degradation of PB-F2. For the first two parts of this project, we propose to work out the involvement of RNF125 (Aim 1) and autophagy/mitophagy (Aim 2) in RIG-I induced viral protein degradation in details. This part will help us understand how innate immunity may fight off IAV infection by induce viral protein degradation.
Previously we found that human PB1-F2 has a three-time longer protein half-life than avian PB1-F2 in human cells. We hypothesize that it may due to their differential responses and promptness to host-specific or signaling-activated protein degradation pathways. For the last part of this proposal, we hope to understand if PB1-F2 and M2 with different host origin response to protein degradation differently in either human or avian cell lines (Aim 3). This part will help us understand the factor deciding viral host range.
Three specific aims are as follows:- Understand how RNF125 modulates influenza virus during infection
- Study the involvement of autophagy/mitophagy in viral protein degradation
- Comparing the promptness of PB1-2 and M2 with either human or avian origin to proteolysis